Introduction:
Sickle cell disease (SCD) causes a hypercoagulable state characterized by frequent thrombotic sequelae including pulmonary embolism (PE) (Connes et al. Blood. Rev. 2016). Although the coagulation cascade is well characterized in SCD, the engineering-based, biomechanical structural properties of sickle blood clots are poorly understood. Furthermore, the role of clot mechanics in both traditional thrombotic disease manifestations (in situ blood clot, PE) but also common sickle cell related sequelae (pain, stroke) is unknown. Since blood clot embolization is inherently a mechanical process, where clots embolize or “fracture” when hemodynamic forces exceed the internal “strength” of the clot (Tutwiler et al. Sci. Adv. 2020), we submit that investigating the mechanical properties of sickle cell blood clots will reveal insights into the disease-specific factors at play. We hypothesize that SCD clots are not only structurally abnormal, but that this abnormality informs an increased propensity for clot embolization and causes severe vascular complications.
Methods:
Under the guidance and approval of the IRB, we acquired venipuncture-derived human blood samples from 10 healthy control subjects (14.3±0.9 g/dL Hgb) and 10 patients with sickle cell disease (7 HbSS, 2 HbSC, 1 HbS beta; 10.5±2.3 g/dL Hgb). We coagulated blood into 3D-printed molds designed for uniaxial extension on our mechanical testing machine, see figure 1. To do so, we added calcium chloride to a concentration of 20mM to reverse the ACD anticoagulant and incubated samples for 60 minutes at 37°C and atmospheric oxygen conditions (Sugerman et al. Curr. Protoc. 2021). Samples were then mounted to our tensile testing machine (Instron, Norwood, MA, USA) and two tests were conducted: extension-to-failure in a simple pure shear geometry and in a mode-I fracture geometry. From this data, we calculated various mechanical metrics by quantifying the structural abnormalities and propensity for embolization of SCD blood clots.
Results and Conclusion:
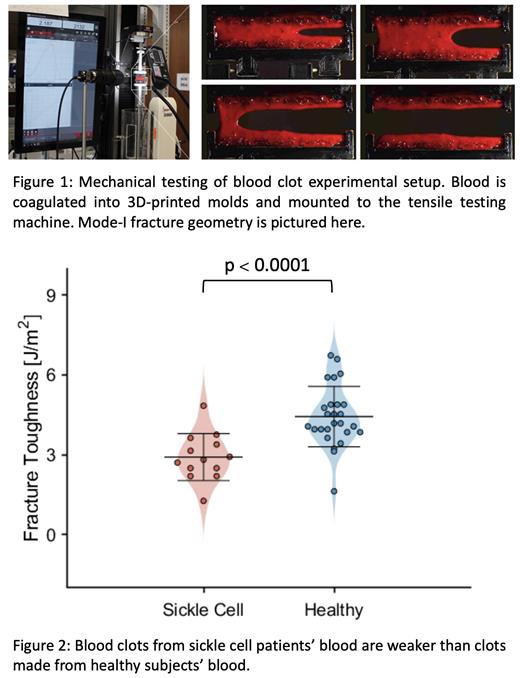
We primarily compared the stiffness and fracture toughness of sickle cell and healthy blood clots. Stiffness measures how the blood clot deforms in response to force, while fracture toughness measures whether a blood clot is strong or weak (i.e its resistance to rupture). The measured stiffness of SCD clots is 7.27±1.68 kPa, which is significantly higher than the stiffness of healthy clots, 5.22±1.09 kPa (p < 0.001). Additionally, the blood from SCD patients makes clots with a fracture toughness of 2.91±0.85 J/m 2, which is significantly lower than the fracture toughness of healthy clots, 4.43±1.11 J/m 2 (p < 0.0001), see figure 2.
The high stiffness of sickle cell blood clots suggests that they are more resistant to deformation than healthy clots. However, the low fracture toughness of sickle cell blood clots suggests that they are more likely to break or embolize when compared to healthy clots. In other words, sickle blood clots exhibit mechanical properties similar to ceramic which has high stiffness and low toughness as opposed to leather (low stiffness, high toughness) or diamond (high stiffness, high toughness).
In conclusion, our biomechanical findings of higher stiffness and lower toughness in sickle blood clots are well aligned with established rheological data showing that SCD red blood cells are less deformable than healthy red blood cells (Lu et al. Exp. Biol. Med. 2020). That is, the low deformability of red blood cells in SCD is apparent even at the clot level, which may inform novel disease mechanisms. The sickle cell related determinants of these biomechanical abnormalities, their role in clinical outcomes (PE, pain and stroke) and their response to therapy is the object of future study.
Disclosures
Rausch:Edwards Lifesciences: Consultancy.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal